THE WHAT? L’Oréal USA has announced that it has reached carbon neutrality for scopes 1 and 2 emissions for all 25 US sites across 12 states, including its manufacturing and distribution facilities, administrative locations and research and innovation sites.
THE DETAILS The US subsidiary hit its goal four years ahead of schedule, the French beauty giant said, the group having set a sustainability commitment to reach carbon neutrality across all sites worldwide by 2025.
L’Oréal USA took a four-pronged approach in order to achieve carbon neutrality comprising optimising energy consumption, producing renewable energy on site, purchasing RECs from local suppliers and procuring renewable natural gas from landfill gas projects.
THE WHY? Stéphane Rinderknech, President & CEO, L’Oréal USA, reveals, “L’Oréal’s sustainability ambition is a transformational effort that has touched every department, becoming a source of pride, inspiration and education for all our 11,000 US employees. Our brands and products are some of the most recognizable in the world, and more than two out every three products we sell in the United States are manufactured here. Our customers can be proud their products are made in facilities that use 100 percent renewable energy. While we are proud of these achievements, we know this is not enough to meet the moment we are in today and must push ourselves ever farther to meet the climate crisis head-on.”
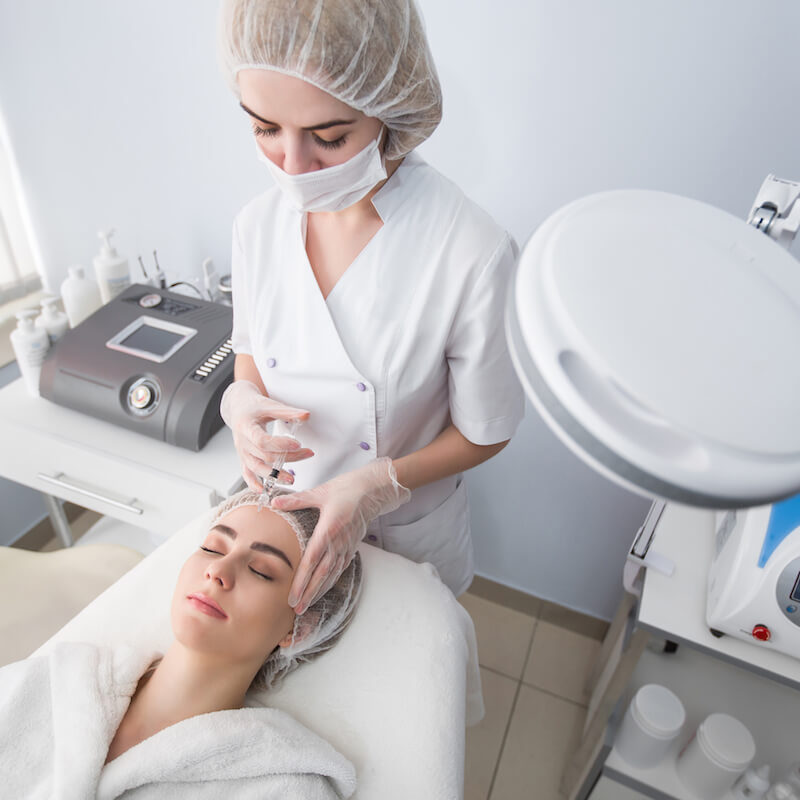
Aesthetic medicine products are developed and regulated to meet stringent safety and efficacy standards. They are typically administered by trained healthcare professionals such as dermatologists, plastic surgeons, and specialized nurses in clinical settings. These products aim to provide effective solutions for cosmetic enhancement, skin rejuvenation, and overall aesthetic improvement, contributing to both physical appearance and self-confidence.
Key categories of aesthetic medicine products include:
-
Injectables: This category includes products such as dermal fillers, botulinum toxins (e.g., Botox), and collagen stimulators. These injectables are used to smooth wrinkles, add volume, and improve facial contours.
-
Skin Rejuvenation Treatments: Products like chemical peels, microdermabrasion systems, and laser devices are used to improve skin texture, reduce pigmentation irregularities, and enhance overall skin tone.
-
Skincare Products: These include medical-grade cleansers, moisturizers, serums, and topical treatments containing active ingredients like retinoids, antioxidants, and growth factors. They are formulated to address specific skin concerns such as acne, aging, and hyperpigmentation.
-
Hair Restoration Products: Medical treatments and products designed to promote hair growth and treat conditions such as male and female pattern baldness.
-
Body Contouring and Fat Reduction: Devices and products used for non-surgical body sculpting, such as cryolipolysis (cool sculpting) devices and injectable lipolytics.
-
Cosmeceuticals: High-performance skincare products that bridge the gap between cosmetics and pharmaceuticals, often containing potent ingredients with proven clinical benefits.
-
Wound Care and Scar Management: Products like silicone sheets, gels, and advanced wound dressings used to improve healing and reduce the appearance of scars.