THE WHAT? IFF has announced that its Chairman and CEO, Andreas Fibig, plans to retire after a 10-year tenure with the company. His departure is slated for early next year.
THE DETAILS Fibig will remain in situ until a successor is named and stay on for a period of time to support a seamless transition; IFF’s Board of Directors is currently evaluating candidates with the support of a leading executive search firm.
“Over the past several years, Andreas has successfully led IFF through some of the most significant milestones in the company’s history,” said Ed Breen, Lead Independent Director of IFF. “IFF has benefited from his experience and commitment to transformation that redefines the possibilities for our industry. Our future is bright as IFF is well-positioned to drive long-term value creation for all stakeholders. The Board and I are grateful for everything Andreas has helped IFF accomplish. We wish him all the best and appreciate his ongoing leadership to ensure a smooth transition as we search for his successor.”
THE WHY? Andreas Fibig, Chairman and CEO, explains, “Leading this 132-year-old iconic company through a true transformation this past decade has been an honor. With the company delivering strong sales growth and the integration of Nutrition & Biosciences well underway, I feel now is the right time to let the next chapter of IFF’s legacy begin. We have created an unrivaled platform that is a true innovation partner to our customers and instilled a real purpose within our company to become a global leader in sustainability. Of all we have accomplished at IFF, what I am most proud of is that IFF has returned to its rightful place as the clear leader in our field, extending its illustrious legacy and poised to define the evolution of our industry for years to come. IFF has never been in a better position, and I look forward to watching its next chapter unfold.”
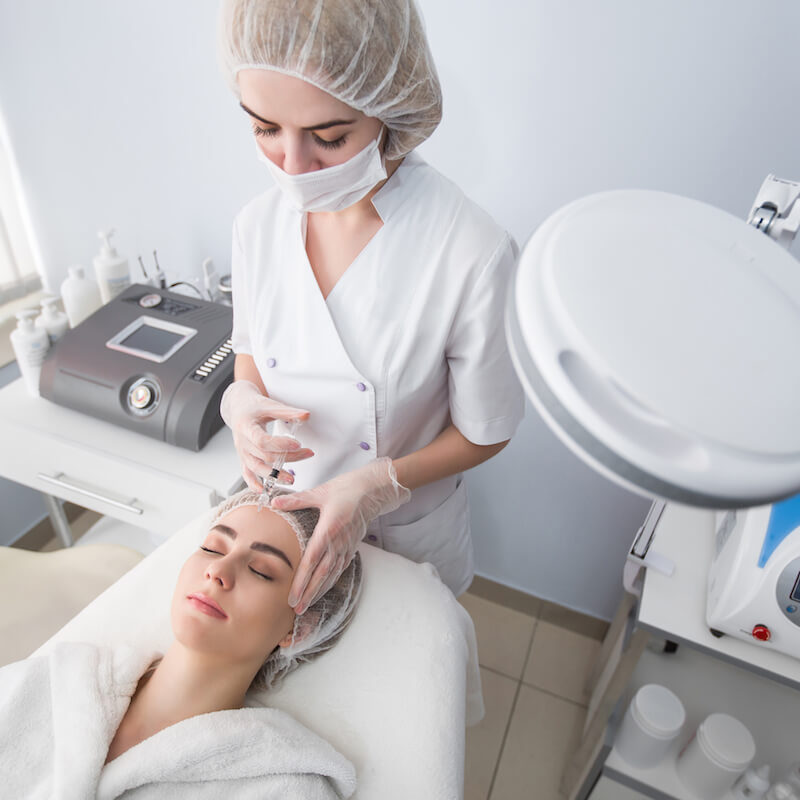
Aesthetic medicine products are developed and regulated to meet stringent safety and efficacy standards. They are typically administered by trained healthcare professionals such as dermatologists, plastic surgeons, and specialized nurses in clinical settings. These products aim to provide effective solutions for cosmetic enhancement, skin rejuvenation, and overall aesthetic improvement, contributing to both physical appearance and self-confidence.
Key categories of aesthetic medicine products include:
-
Injectables: This category includes products such as dermal fillers, botulinum toxins (e.g., Botox), and collagen stimulators. These injectables are used to smooth wrinkles, add volume, and improve facial contours.
-
Skin Rejuvenation Treatments: Products like chemical peels, microdermabrasion systems, and laser devices are used to improve skin texture, reduce pigmentation irregularities, and enhance overall skin tone.
-
Skincare Products: These include medical-grade cleansers, moisturizers, serums, and topical treatments containing active ingredients like retinoids, antioxidants, and growth factors. They are formulated to address specific skin concerns such as acne, aging, and hyperpigmentation.
-
Hair Restoration Products: Medical treatments and products designed to promote hair growth and treat conditions such as male and female pattern baldness.
-
Body Contouring and Fat Reduction: Devices and products used for non-surgical body sculpting, such as cryolipolysis (cool sculpting) devices and injectable lipolytics.
-
Cosmeceuticals: High-performance skincare products that bridge the gap between cosmetics and pharmaceuticals, often containing potent ingredients with proven clinical benefits.
-
Wound Care and Scar Management: Products like silicone sheets, gels, and advanced wound dressings used to improve healing and reduce the appearance of scars.