THE WHAT? Johnson & Johnson has announced a plan to spin off its consumer health business, creating a new publicly traded company.
THE DETAILS The new Consumer Health Company would comprise four US$1 billion megabrands and 20 brands over US$150 million, including Neutrogena, Aveeno, Tylenol, Listerine, Johnson’s and Band-Aid. It is expected to generate revenue of US$15 billion in full year 2021/2 and its Board of Directors and executive leadership is yet to be determined or announced.
Following the planned separation, Alex Gorsky will serve as Executive Chairman of Johnson & Johnson and transfer the CEO role to Joaquin Duato, effective January 3, 2022.
THE WHY? Gorsky explains, “Throughout our storied history, Johnson & Johnson has demonstrated that we can deliver results that benefit all our stakeholders, and we must continually be evolving our business to provide value today, tomorrow and in the decades ahead. Following a comprehensive review, the Board and management team believe that the planned separation of the Consumer Health business is the best way to accelerate our efforts to serve patients, consumers, and healthcare professionals, create opportunities for our talented global team, drive profitable growth, and – most importantly – improve healthcare outcomes for people around the world…
“We believe that the New Consumer Health Company would be a global leader across attractive and growing consumer health categories, and a streamlined and targeted corporate structure would provide it with the agility and flexibility to grow its iconic portfolio of brands and innovate new products. We are committed to the success of each organization, as well as our company’s more than 136,000 employees around the globe, who will remain the backbone of these businesses.”
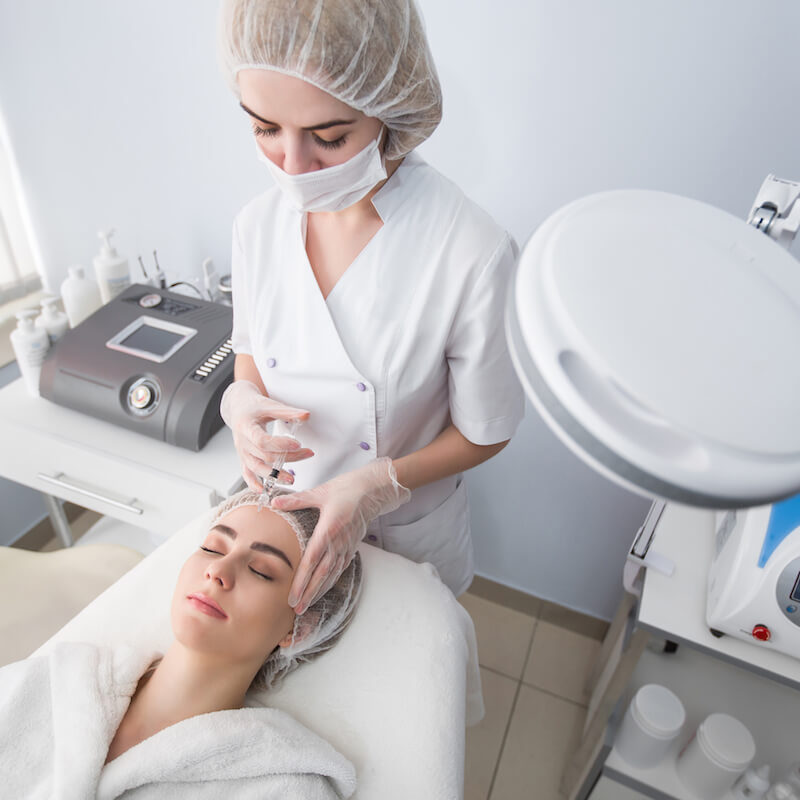
Aesthetic medicine products are developed and regulated to meet stringent safety and efficacy standards. They are typically administered by trained healthcare professionals such as dermatologists, plastic surgeons, and specialized nurses in clinical settings. These products aim to provide effective solutions for cosmetic enhancement, skin rejuvenation, and overall aesthetic improvement, contributing to both physical appearance and self-confidence.
Key categories of aesthetic medicine products include:
-
Injectables: This category includes products such as dermal fillers, botulinum toxins (e.g., Botox), and collagen stimulators. These injectables are used to smooth wrinkles, add volume, and improve facial contours.
-
Skin Rejuvenation Treatments: Products like chemical peels, microdermabrasion systems, and laser devices are used to improve skin texture, reduce pigmentation irregularities, and enhance overall skin tone.
-
Skincare Products: These include medical-grade cleansers, moisturizers, serums, and topical treatments containing active ingredients like retinoids, antioxidants, and growth factors. They are formulated to address specific skin concerns such as acne, aging, and hyperpigmentation.
-
Hair Restoration Products: Medical treatments and products designed to promote hair growth and treat conditions such as male and female pattern baldness.
-
Body Contouring and Fat Reduction: Devices and products used for non-surgical body sculpting, such as cryolipolysis (cool sculpting) devices and injectable lipolytics.
-
Cosmeceuticals: High-performance skincare products that bridge the gap between cosmetics and pharmaceuticals, often containing potent ingredients with proven clinical benefits.
-
Wound Care and Scar Management: Products like silicone sheets, gels, and advanced wound dressings used to improve healing and reduce the appearance of scars.